Test Kits For Safer Sex
ClubHombre.com:
-Men's Health-:
-Safe Sex:
Test Kits For Safer Sex
| |
Subtopic |
Posts |
|
Updated |
 | Archive 01 | 50 | |
2006/01/31, 11:32 pm |
I have seen them on E-Bay recently but not now. Look at ebay.com from time to time and do a search for HIV Test. You will find the ones that arent USA approved yet.
| By Sabio on Sunday, April 18, 2004 - 01:54 pm: Edit |
HIV infection and testing time frames:
Here is a summary of how the AIDS virus and antibodies evolve after the initial infection, and how this affects different test results and infectiousness to others.
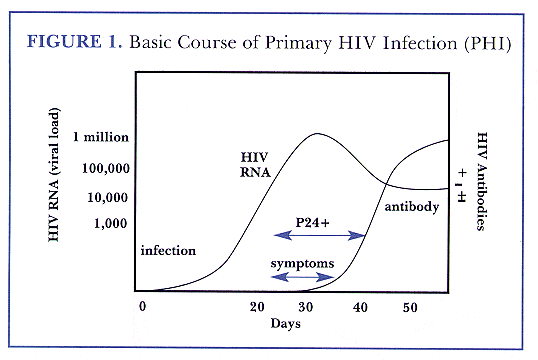
Stages:
1. After the onset of infection, the virus starts multiplying and typically reaches levels that can be detected by the DNA/PCR test within 10 days.
2. The virus continues to multiply, unchecked, for another 3 weeks or so. The viral load (amount of virus) becomes very high during this period. Flu-like symptoms start to develop, and the person becomes more infectious than typical. There are RNA tests to measure the viral load.
3. The body finally catches up with the situation and starts producing antibodies to fight the virus. These antibodies are what the most common HIV tests (Elisa, OraQuick, etc.) detect, hence these tests do not work until about one month after infection. Antibodies push the viral load (hence infectiousness) down to a steady-state level.
4. Antibodies keep fighting the virus in this steady state for few to several years, with no visible symptoms.
5. Eventually, the virus will have caused enough damage to the immune system that the person reaches the stage of formally having AIDS, as opposed to just being HIV+.
Remarks:
1. If someone is getting tested because they are worried about having been exposed to the virus, testing guidelines recommend a long wait before taking the exam (3 weeks for the DNA/PCR test, 3 to 6 months for the antibody tests). The reason is that the person would want a negative result to be almost certain, therefore the waiting period is chosen such that the overwhelming majority of infected people, not just for the majority, would test positive at that point.
2. On the other hand, if someone is going to have sex right away and gets tested to reduce the probability of infecting others (as in the p4p test kit scenario), the test will provide some information much earlier. A positive result is almost always correct, and a negative result is probably correct in 10 days for DNA/PCR and in a little over a month for antibody tests.
3. Higher viral load means higher infectiousness, but not in direct proportion. It is close to a cubic root rule, with the infectiousness approximately doubling for each 10-fold increase in viral load.
4. Therapies help reduce the viral load, quite significantly in some cases. The timely start of therapy is one of the benefits of getting tested early. Not only does it help the patient's health, but it also reduces his/her infectiousness to others.
References for this information are included in the above posts, mainly in the March 30, 2003 post.
| By Sabio on Wednesday, April 28, 2004 - 11:28 pm: Edit |
Percentages of HIV+ prostitutes around the world, according to US Census Bureau. The numbers are higher than I expected, for example 18% in Sao Paulo as of 1998.
Full reports
Tables
Maps
International site
There is also information about HIV statistics in other segments of the population.
Sabio
Can you comment on whether that 18% figure includes intravenous drug users who are also prostitutes -- not an unusual combination particularly among lower income classes (though not limited to them)?
Since the HIV rate for IVD users is VERY high, might it not be skewing results among prostitutes upward to the 18 percent overall e.g. particularly if the rate for IVD/Prostitutes is, say, in the 60th percentile.
I'm not an expert in interpreting these reports/tables, but it seems they don't provide important details such as the one to which I refer.
| By Sabio on Thursday, April 29, 2004 - 01:43 pm: Edit |
SF_Hombre
I agree with you that the 18% includes prostitutes who got infected through IV drug use, since it is an overall number. I also agree that this will tilt the numbers up, since the probability of catching HIV from an infected needle is drastically higher than getting it from sex. If we can recognize who among the providers is an IV drug user and who is not, we can reduce our risk.
The trends reported by the Census Bureau are also unfavorable. In Rio de Janeiro, the HIV infection rate among prostitutes was 3% in 1987, and became 11% in 1993. I could not find more recent numbers. The maps of HIV infection in the above link also show significant variability in concentrations within each country.
| By Sabio on Sunday, June 27, 2004 - 02:29 pm: Edit |
One step closer to OTC?
US government expands the use of oral HIV test OraQuick
The Bush administration said Friday it will permit wider use of an oral test for the AIDS virus that gives results in 20 minutes. The relaxed rules will allow screenings in HIV counseling centers, community health centers and doctors' offices.
"HIV testing has never been easier or more accessible than it is today," Health and Human Services Secretary Tommy Thompson said, announcing the changes in advance of National HIV Testing Day on Sunday.
In March, the government approved the OraQuick test for use mainly in hospitals and large health clinics. The test is manufactured by OraSure Technologies Inc. in 38,000 laboratories and, unlike other tests, does not require blood. With OraQuick, a technician wipes a treated cotton swab along the gums, picking up not saliva but cells lining the mouth.
The swab is placed in a vial, and infection is signaled by the presence of reddish-purple lines in a window on the vial.
I am really curious how they arrived at the 18% HIV infection rate for sex workers in Rio/Sao Paulo.
Who exactly did they test? Girls in L'Uomo? Help?
Probably girls with STD infection that were poor and went to free public health posts. Even then, 18% looks too high to me.
About OraQuick>> anyone can get hold of Oraquik tests? I would love to get a few of them for my own use
Has anyone tried these test kits? They are made in Canada and will ship to U.S.
http://www.htkits.com/
| By Sabio on Monday, August 09, 2004 - 02:43 pm: Edit |
Pilotboy:
I looked at the web site and could not find information about FDA approval. Do you know if they are approved?
All the OTC kits that I know of are not FDA-approved. They sometimes state that FDA would not approve DIY HIV tests. While the statement is true, I am wary that it may be a convenient excuse to avoid independent verification of the product's claims.
The FDA does not approve them. Actually, they approved OraQuik for use in laboratories for instant testing, not for you to take home.
Bush does not want to make sex any safer.
I would love to find OraQuik. Anyone can bring some to Brazil??
Other tests would be good if approved by other maior government agencies in Europe, Canada, Japan. Any recommendations??
Actually, the FDA DOES approve OTC HIV tests to be sold in the U.S. but thus far has not approved any. This does not prevent the manufacturer from selling the tests OTC in other countries, since the sales of same are regulated by those respective countries' regulatory agencies. (For example, Thailand has their own FDA that governs HIV tests). On the other hand, the U.S. FDA has approved a home collection kit for HIV testing (Home Access HIV-1 Test System) where the customer can collect a blood sample (actually a blood spot blotted on an absorbent pad), send it off for a lab to be tested and call in anonymously to get the results.
By the way, sales of the Oraquick saliva test have apparently been suspended by the manufacturer (Orasure Technologies) because of false positive problems, but no word on when they will be selling again.
| By Sabio on Thursday, August 12, 2004 - 02:42 pm: Edit |
False positives are bad news for OraQuick, as they always claimed almost 0% false positives (versus 0.4% false negatives). Could you please give us a reference for this news?
BTW, the FDA prohibition on DIY kits that I was talking about applies to tests where you get the result by yourself. The rationale is the need for a professional counselor in case of a positive result, which they have for those home tests where you send the sample to the lab. However, the US HHS secretary said that they may change this policy in the future.
Thank you for the information.
Finally got my hands on the Oraquick Advance HIV test. $28. each from this site. http://www.bsafehiv.com/
It says right on the package for sale outside the U.S
A 10 year old could follow the simple directions. Good thing our politicians are looking out for us, huh?
BS - if you want me to bring any, I come down again March 5th.
Let's say you're in the bedroom with some chick, she takes the test and passes. Does that mean you feel more comfortable barebacking her?
What if she fails? Do you do her anyways with protection? Do you tell her the results? If I had to tell a hooker she was HIV, I'm not sure I know the proper way to go about doing so.
Thanks for posting Pilotboy. Does anyone know about an inhome STD screening kit?
Nice questions Deeg.
First of all, the test cannot tell if she is HIV postive, just whether she was HIV+ 3 months prior ot the test.
Second, Deeg touches on some very important material. Testing is a very personal and sometime stressful occasion. Let's say you admin the test-- that is a exlosive possible siutuation. Are you ready and qualified to cousel her if +? I suggest leaving testing and such to professionals-- if you insist on getting someone tested bring them to a clinic.
It's a great idea for testing yourself tho.
Check out Redsnake.com there is some info on STD kits.
definitely, I am interested in getting some tests. Please contact me whoever gets a few of them and is heading towards here ........
Most of these oral and piss tests claim good efficacy.. Sorry to break it to you but the false positive rates on these tests are significant enough to render them useless. Only the blood tests are reliable. The saliva and urine tests are going to be used in third world nations due to lower costs. They are a decent test for seeing if you have HIV.. If you come up positive they will then give u a blood test to make sure u do not have a false positive. These tests are stressful enough. You may just give yourself a heart attack by taking a 20 dollar test that is unreliable.. Just my two cents. For those of you interested there is a scam stock on the AMEX ticker HIV formerly known as calypte that makes the piss tests.. A total joke.
It's virtually impossible to get an HIV test (whether blood, oral or urine) approved by the FDA if it doesn't yield a sensitivity and specificity of at least 98% (read false negative and false positive rates of less than 2%). Oraquick is FDA approved, and in testing thousands of subjects, those results were sustained, including independent evaluations by the FDA. Having said that, they recently have been having false positive problems in a few locations and are investigating, as is the FDA, so who knows, they may be pulled off the market.
The CDC has been very big in pushing these "alternate" tests to blood, because it provides a way to get testing to a lot of people who might not otherwise be willing to be tested, particular outside the U.S. where there are taboos and superstitions about blood letting. Using a test with an accuracy of at least 98% can do a world of good in some settings, particularly if it increases testing among people who might not otherwise be tested, but of course, if you're one of the 1-2% who get's the wrong result, it can be a nightmare for you. It's worth noting that for any rapid test, manufacturers state that the results should be confirmed, and not rely solely on the rapid test results. This typically will include a blood test, using more complex laboratory tests, such as EIA's, IFA's and/or Western blots. These tests also take a lot longer to get results.
There are a lot of scams out there for HIV tests, most sold over the internet, but there are also tests that the FDA has approved for alternate fluids, such as saliva and urine that are not scams. For a list of HIV tests that have been approved by the FDA, go to http://www.fda.gov/cber/products/testkits.htm.
Of course, there are tests that the FDA has not approved that are good, mainly because they simply aren't sold in the U.S., but as a start, this list will tell you who some of the legitimate players are. This list is updated regularly by the FDA as new tests are approved.
| By Sabio on Tuesday, December 27, 2005 - 02:36 pm: Edit |
Sojourner:
Thank you for your posts. I looked into the false positives problem and the article below addresses it. Strange that it happened in one area, and that the number of cases is so significant.
I hope they get to the bottom of this, as it is a make-or-break issue for test kits. The FDA is currently processing the OTC application for OraQuick, so they'd better investigate these false positives. Here is the article (abridged):
Priya David Reports
December 9, 2005
SAN FRANCISCO -- A promising new oral HIV test that uses fluid swabbed from the mouth to quickly and easily detect the virus that causes AIDS incorrectly diagnosed a quarter of the people who tested positive in San Francisco, city health officials found.
Forty-seven people who tested positive after using the OraQuick Advance HIV test in city clinics were not infected at all, the San Francisco Department of Public Health said this week.
Investigators learned of the errors after follow-up blood screenings gave the patients a clean bill of health, and health officials stopped using the test at City Clinic, the health department's primary testing location for HIV.
At the same time, the U.S. Food and Drug Administration, which approved the OraQuick test for professional use last year, is now considering a request from drug maker Orasure Technologies, of Bethlehem, Pa., to approve it for home use and over-the-counter sales.
"We need to vigorously look at this," said Jeffrey Klausner, San Francisco's director of sexually transmitted disease prevention and control services. "You wouldn't want to have a home test with this problem."
Klausner said there are no known instances in which the test missed an HIV infection that a traditional blood screening would have caught.
Orasure Technologies chief executive Douglas Michels said San Francisco is the only city that has reported the problem, and data collected from thousands of oral tests conducted in cities across the country show the test is reliable.
A statewide survey by the California Department of Health Services that was prompted by the San Francisco findings has not yet found a similar problem.
The web address given is not good at this date. A search of the CDC web site for "AIDS Home Test Kits" yielded this:
"However, there is one HIV-1 Home Collection Test System that is currently approved by the FDA in which a sample for testing is collected in the privacy of your home and then sent to a laboratory for analysis. The "Home Access Express HIV-1 Test System" manufactured by Home Access Health Corporation is the only HIV-1 Home Collection Test System approved by FDA and legally sold in the United States.
"Be aware that there are a number of different HIV home test systems and kits that are being marketed on the Internet and through magazine or newspaper promotions that claim to detect antibodies to HIV in blood or saliva samples and provide results in the home in 15 minutes or less. The FDA has NOT APPROVED these rapid HIV-1 home test kits being promoted on the Internet for use and marketing in the United States. Some of the HIV home test kits falsely claim to be approved by the FDA or manufactured in a FDA approved/registered/licensed facility. All HIV home sample collection kits approved to date by FDA require laboratory analysis and provide counseling for the consumer.
"FDA warned consumers about an unapproved, fraudulently marketed home-use HIV test system labeled "Lei-Home Access HIV test" distributed by Lei-Home Access Care located in Sunnyvale, California in a press release issued on September 26, 1997. The "Lei-Home Access HIV test" was advertised on the Internet as the "Personal HIV Test Kit" and was offered for sale through several Central Valley pharmacies. After an extensive investigation by FDA, the businessman responsible for distributing this fraudulent HIV test kit was recently sentenced to over 5 years in prison for selling the medically useless HIV test kits to consumers in the United States."
[MORE....]
Sabio,
Just as a point of clarification, Orasure has not filed a formal application for an Over the counter application for their Oraquick product at this time. The press releases you have read pertain to a meeting they had with an FDA Advisory Panel to seek input from this group on that possibility. Of course, many in the market and in the press do not understand this distinction, but it does certainly suggest they are certainly interested in making such an application. Whether or not the information they received from that meeting means they are still interested in making such an application remains to be seen. I have no personal information in that regard, I'm just familiar with the regulatory process.
Hunterman,
You are correct that Home Access has FDA approval to market an HIV-1 Home Collection Test System, but what the web address is nonetheless up to date. They can sell the system that allows for the collection of specimens "over the counter", but what the user buys is not a test itself. Note that they call it a "Test System" not a "Test". This is a fine distinction, granted, but the system includes the test material itself that the consumer does not physically received. They collect a blood specimen, and mail it in to Home Access. Home Access then uses an FDA approved HIV test (one of the ones approved by the FDA and listed on the web site I gave you) to obtain a test result, which is then communicated back to the buyer when he calls in by phone. The information you quote from the CDC is correct, however the CDC does not approve HIV tests in the U.S. It does participate in evaluations, and on occasion, formal clinical studies of HIV tests, but even if the CDC wants to sell an HIV test for diagnostic purposes, it would have to gain FDA approval. Having said that, the CDC (and its researchers) does on occasion provide HIV tests that are for "investigational use only" or "research use only" to certain labs, but these must comply to FDA regulations, and cannot be used for diagnosis. (They typically are run side by side with approved diagnostic HIV tests).
The website lists all approved HIV tests, and if the test is not on the website, it can not be legally sold in the U.S. (including to Home Access's testing laboratories). From my experience, the FDA usually updates it within days of when a new test is approved (or approval withdrawn), so it is as reliable a source as you will find on the internet of U.S. approved HIV tests.
As a followup to Sabio's post above on the problems with false positives with the Oraquick Rapid HIV test, the CDC has completed it's investigation of the reported "false positive" problem in the U.S., where a small number of sites experienced this problem. They concluded that the test performs acceptably (false positive rate well under 1%) and blamed the problem either on problems at the site specifically, or something unusual in the populations that were being tested, not the test itself. The full article can be found at the following webpage, and has been pasted below as well.
http://www.medpagetoday.com/InfectiousDisease/HIVAIDS/tb/2631
===========================================================================================
CROI: CDC Finds Rapid HIV Test Meets FDA Accuracy Criteria
By Ed Susman, MedPage Today Staff Writer
DENVER, Feb. 7 - A rapid HIV diagnostic test has performed just as advertised, according to a CDC study undertaken in light of recent reports questioning the test's accuracy, researchers reported here.
The study found nothing wrong with the tests or the overall results, Bernard Branson, M.D., a medical epidemiologist with the CDC, told a press conference during the opening day of the 13th annual Conference on Retroviruses and Opportunistic Infections.
"As with all screening tests, false-positive rapid HIV tests will occur and should be expected," said Dr. Branson. "Confirmatory testing after a reactive test must always be performed."
Dr. Branson said the CDC has been conducting post-marketing surveillance since 2003 after the FDA approved the OraQuick Rapid HIV-1 Antibody Test that can determine whether a person is HIV positive from a blood sample by fingerprick or by oral fluid swabbed from the mouth.
The tests can be performed with either a needlestick to obtain a blood sample; or a swab of the outer surface of the upper and lower gums with the flat pad of the test device to obtain an oral fluid sample. The sample is then inserted into a reagent and after 20 to 40 minutes the device registers a line which indicates if the sample is negative or positive.
Dr. Branson said that in four prospective studies, the OraQuick blood tests reported 12 false positives when it was used to test 12,010 reference negative samples -- a specificity of 99.9%; the same samples were tested with the OraQuick oral fluid and 54 false positive occurred, a specificity of 99.6%.
CDC researchers reviewed sites that were apparently finding an excess of false positives, such as at three sites in New York, including one clinic where 32 out of 1,581 tests produced a false positive results -- a specificity of 98%. However, when the CDC reviewed tests at seven other sites in New York, the tests proved to have a 99.9% specificity.
In San Francisco, where specificity at one clinic was as low as 96.3% -- six false positives in 160 tests -- a CDC sampling of 11 sites produced five false positive out of 551 tests, or a specificity of 99.1%.
"Excessive false-positive at these sites appear to be related to unidentified site- or host-specific factors," Dr. Branson concluded in his report. He said he could find no evidence for a lot-related or device problem.
"OraQuick specificity is slightly lower with oral fluid than with whole blood, but well above the FDA's minimum threshold of 98% with both specimen types," Dr. Branson said.
Harold Jaffe, M.D., a professor of public health at Oxford University in England, and a former director of the CDC's National Center for HIV, STD, and TB Prevention, said, "My take is that these tests are working very well, but it is important to pursue an investigation into the areas where there were higher than expected false positives to determine if there is something about how the tests are conducted or who is administering the tests that is causing a problem."
Dr. Branson said a review of the procedures and quality assurance is essential if sites experience excess false-positives, and those probes are underway. He said other factors are also being investigated such as collection of too much specimen and over-interpretation of the results by the test operators.
| By Buckho1 on Saturday, October 30, 2010 - 11:53 pm: Edit |
If your concerned about HIV. Then you might want to check out this web page. They have instant and accurate tests for sale. Even though there can be a 30 to 90 day gestation period this test is a lot better then nothing and because its a blood test much more accurate then a Oral or urine test. If you like bareback or break a condom.
WWW.surehivtest.com
| By Laguy on Sunday, October 31, 2010 - 12:04 am: Edit |
Three posts in total by this guy, all SPAM and identical SPAM at that. Am I imagining things, or does it seem whenever we get a SPAMster on this site, they post in threes?
| By Laguy on Sunday, October 31, 2010 - 12:06 am: Edit |
Whoops, this guy is now up to four, at least as of this second. Hopefully, he will get booted for his obnoxious SPAMster behavior.
| By Buckho1 on Sunday, October 31, 2010 - 12:47 am: Edit |
Laguy, I don't know what your problem is ? All post were in the proper threads and only said if your interested in this you can look. I can only guess your not interested. Don't look, cover you eyes and ears as well.
Buckho1
Yeah! Lets kill him!!
(Angry Villiagers with Torches)
I think its a valid concern considering your account was created mmmm today..? And you only have 5 posts 4 of which are promotional..
Personally I don't care, but La does have a point..
| By Buckho1 on Sunday, October 31, 2010 - 01:04 am: Edit |
I'm sticking with my thinking, you may not care however someone else out there may care a great deal. No one is forcing you to do anything you don't want to. It's just information, don't be afraid of new information. It can't hurt you if you don't look or believe it.
Buckho1
"Yeah! Lets kill him!!
(Angry Villiagers with Torches)"



| By Laguy on Sunday, October 31, 2010 - 02:14 pm: Edit |
Well, at least three of his four identical SPAM posts (originally posted in four different threads)have since been removed by the administrator of this site.
(Message edited by LAguy on October 31, 2010)
On a more serious note, these kind of tests do seem pretty revolutionary, but I wonder how reliable they are..
Having never used one it would be really interesting to hear from those that successfully used this. Even more so.. what the dialogue between you and the girl was that lead up to administering the test?
"So...you don't have Aids do you.......?"
Curious to hear what smooth talking took place to pull that off..Especially the one involving blood.. Seems like a tough sell..
Genuinely interested..
J666


![]()
![]()
![]()